Introduction
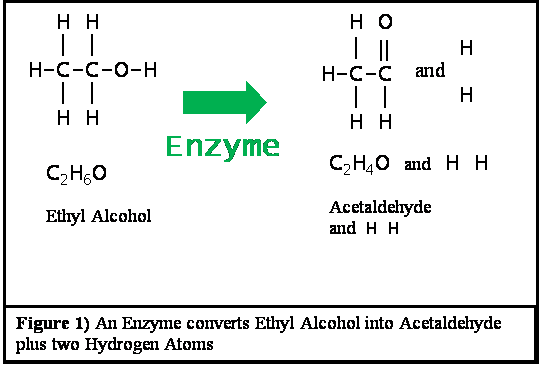
When you drink beverage alcohol around 2 to 8 percent is lost through urine, sweat, or the breath. The other 92 to 98 percent is metabolized by your body. All ethyl alcohol which is broken down in the human body is first converted to acetaldehyde, and then this acetaldehyde is converted into acetic acid radicals--also known as acetyl radicals. Acetaldehyde is a poison which is a close relative of formaldehyde and which we will discuss it in more detail later on. Acetic acid is the essential component of vinegar. The acetic acid radical is the combining form of acetic acid. This acetic acid radical combines with Coenzyme A to form acetyl-CoA. The acetyl-CoA rhen enters the Krebs Cycle, which is the basic powerhouse of the human body. Inside the Krebs Cycle this acetyl radical is eventually broken down into carbon dioxide and water.There are three different enzymes which the body uses to convert alcohol to acetaldehyde. All three of these enzymes work by stripping two hydrogen atoms off from the alcohol molecule. This converts the alcohol molecule into a molecule of acetaldehyde as shown in Figure 1.
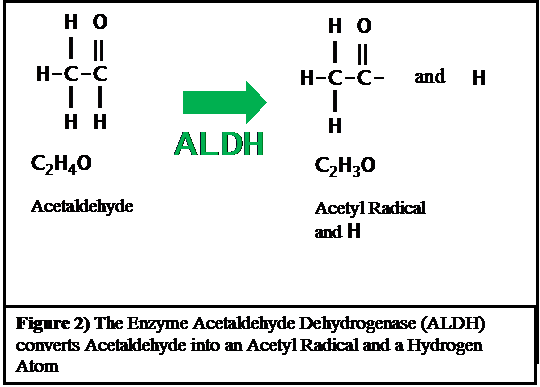
The acetaldehyde is then converted by a different enzyme into the acetyl radical as shown in Figure 2.
Let us take a closer look at the enzymes which convert alcohol into acetaldehyde.
The Three Alcohol Enzymes
The three enzymes which can convert alcohol to acetaldehyde are:- alcohol dehydrogenase (ADH)
- cytochrome P450 (CYP2E1)
- catalase
Alcohol dehydrogenase: The name "alcohol dehydrogenase" sounds like quite a mouthful, but it is quite self-explanatory if we break it down into its component parts. "de-" is a prefix which means "to remove". We find it in such words as "dethrone" which means "to remove from the throne". "-ase" is a suffix which means "enzyme". Any time you see a chemical term which ends in the suffix "-ase" you know that you are dealing with an enzyme. "hydrogen" means "hydrogen" of course. So "de-hydrogen-ase" means "an enzyme which removes hydrogen atoms", and "alcohol dehydrogenase" means "an enzyme which removes hydrogen atoms from the alcohol molecule". The name alcohol dehydrogenase is sometimes abbreviated to ADH.
Alcohol dehydrogenase is the workhorse of the alcohol enzymes--it breaks down the majority of the alcohol that enters the human body. Alcohol dehydrogenase is actually the name for a family of enzymes which break down alcohol--each of which has a slightly different molecular structure. Researchers have identified as many as 10 varieties of the alcohol dehydrogenase molecule. All of them bring about the same chemical reaction--the difference is that some varieties of alcohol dehydrogenase work more efficiently than others. As we shall see below, these variations in the alcohol dehydrogenase molecule can explain why some individuals react differently to alcohol than others.
The alcohol dehydrogenase molecules do their work primarily in the stomach and the liver, although traces of them are found in other tissues as well. The hydrogen which is released when alcohol dehydrogenase turns alcohol into acetaldehyde is bound to a compound called NAD+ (Nicotinamide Adenine Dinucleotide) to form NADH (this is short for Nicotinamide Adenine Dinucleotide plus Hydrogen). Please visit the Wikipedia entry NADH for more information about this compound.
Alcohol dehydrogenase does its work in the cellular fluid (cytosol) of the cell. For more information about this enzyme please visit the Wikipedia entry alcohol dehydrogenase.
Cytochrome P450 2E1 (CYP2E1): In light social drinkers nearly all the alcohol consumed is taken care of by alcohol dehydrogenase as described above. However, the enzyme Cytochrome P450 2E1 (abbreviated CYP2E1) becomes quite active in metabolizing alcohol in chronic heavy drinkers. CYP2E1does its work in the liver. The hydrogen released by this reaction is bound to oxygen and to NADPH to form water and NADP+. This reaction takes energy rather than producing it.
CYP2E1does its work in the microsomes of the cell. This is sometimes referred to as MEOS (Microsomal Ethanol Oxidizing System). CYP2E1 is a member of the Cytochrome P450 enzyme family. Please visit Wikipedia for more information about CYP2E1, Cytochrome P450, and MEOS
Catalase: Catalase is found in tiny organs inside of cells called peroxisomes. Catalase is found all over the human body. When catalase turns alcohol into acetaldehyde the hydrogen which is released is bound to hydrogen peroxide molecules which then become water. Although catalase is active everywhere in the body, catalase is of particular interest to researchers because it metabolizes alcohol in the brain. The acetaldehyde released into the brain by the metabolism of alcohol by catalase has the potential to combine with neurotransmitters to form new compounds known as THIQs (tetrahydroisoquinolines, also sometimes called TIQs). Some researchers believe that THIQs are the cause of alcohol addiction and that the presence of THIQs distinguishes addicted drinkers from social drinkers. Other researches strongly dispute the validity of the THIQ hypothesis of alcohol addiction.. The actual role of THIQs remains controversial and a topic for further research.
Catalase does its work in the peroxisomes of the cell. For more information about this enzyme please visit the Wikipedia entry Catalase.
Summary: Figure 3 summarizes how the three enzymes interact with alcohol to produce acetaldehyde.
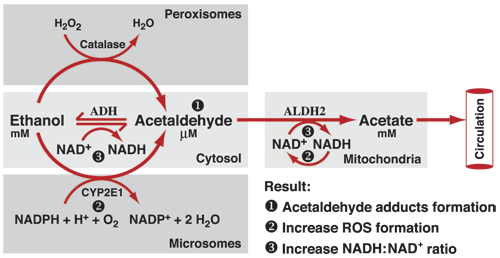
Figure 3) The Actions of the Three Enzymes
How Acetaldehyde Dehydrogenase Works
Acetaldehyde dehydrogenase does its work in the mitochondria of cells and removes a hydrogen atom from acetaldehyde to produce an acetic acid radical as is shown in Figure 2 above. This hydrogen atom combines with NAD+ to form NADH.There are several varieties of aldehyde dehydrogenase found in the human body. The one which normally breaks down acetaldehyde is called ALDH2. There is another variety aldehyde dehydrogenase found in the human body which is called ALDH2*2. ALDH2*2 is only about 8% as efficient as ALDH2 in metabolizing acetaldehyde. Some East Asian people have ALDH2*2 instead of ALDH2 in their bodies. These individuals find the effect of alcohol to be very unpleasant as we discuss below.
The aldehyde dehydrogenase enzymes are found in many tissues of the body, but are at the highest concentration in the liver
The Problem with Too Much NADH
Alcohol metabolism produces excess amounts of NADH (Nicotinamide Adenine Dinucleotide plus Hydrogen). This excess of NADH can lead to acidosis from lactic acid build-up and hypoglycemia from lack of glucose synthesis. It can also lead to weight gain, fatty liver, and heart attack.Alcohol Affects Some People Differently from Others
Women: If a woman and a man of the same weight drink the same amount of alcohol under the exact same circumstances, the woman will on the average have a much higher BAC (Blood Alcohol Content) than the man. This is because women have much less of the enzyme alcohol dehydrogenase in their stomachs than men do. If the same man and woman are given an injection of alcohol instead of drinking it they will tend to have the same BAC. This is because when the alcohol is injected it bypasses the alcohol dehydrogenase in the stomach.East Asians and American Indians: Most individuals use a form of acetaldehyde dehydrogenase called ALD2 to metabolize the acetaldehyde which results from alcohol metabolism. However, many East Asians and American Indians produce a form of acetaldehyde dehydrogenase called ALD2*2 which is far less efficient at breaking down acetaldehyde than ALD2. ALD2*2 is only about 8% as efficient as ALD2 at metabolizing acetaldehyde.
Additionally many East Asians and American Indians have a form of alcohol dehydrogenase that is more efficient at turning alcohol into acetaldehyde than that of people from other genetic backgrounds. The end result is that these people wind up with large amounts of the poisonous compound acetaldehyde in their bodies whenever they drink alcohol. This acetaldehyde causes their faces to flush and leads to headaches, nausea, vomiting, heart palpitations and other extreme physical unpleasantness. This reaction to alcohol is sometimes referred to as the "flush syndrome". The symptoms of flush syndrome are exactly the same as the symptoms caused in people who take the anti-drinking medication antabuse. Antabuse also causes a build-up of acetaldehyde within the body. As many as 50% of people of Japanese descent are estimated to show flush syndrome. Flush syndrome is more severe in some individuals than others. It is estimated that individuals with severe flush syndrome do not develop alcohol problems because they find drinking alcohol to be extremely unpleasant.
Older Males: As men age they tend to produce less alcohol dehydrogenase. Older men are likely to become more intoxicated on smaller amounts of alcohol than younger men. Alcohol dehydrogenase in women is apparently not affected by age.
Menopausal Women: Apparently hormone changes which occur at menopause can cause menopausal women to become more intoxicated on smaller doses of alcohol.
People with Liver Damage: People with liver damage produce less alcohol dehydrogenase than do those with healthy livers and thus can become more intoxicated on smaller doses of alcohol. This phenomenon is referred to as Reverse Tolerance.
Frequent Heavy Drinkers: Frequent heavy drinkers produce more alcohol dehydrogenase than other people and thus become less intoxicated on larger quantities of alcohol. These people can metabolize up to 38 ml (over 2 standard drinks) of alcohol per hour whereas the average person metabolizes only around 13 ml (about 0.7 standard drinks) per hour.
How Antabuse Works
Antabuse is the drug that makes people sick if they drink alcohol. The drug antabuse binds to the enzyme acetaldehyde dehydrogenase and prevents it from breaking down the acetaldehyde produced by the metabolism of alcohol. Since acetaldehyde is a poison, as it builds up it produces very unpleasant symptoms including facial flushing, headaches, nausea, vomiting, heart palpitations and other extreme physical unpleasantness. Large quantities of alcohol mixed with antabuse can lead to death.Why You Shouldn't Drink on an Empty Stomach
The surface area of the human stomach is only a couple of square feet, but because the small intestine has protrusions called villi, the surface area of the small intestine is thousands and thousands of square feet. Because of this fact the small intestine is many, many times more efficient than the stomach at absorbing alcohol. If you want the alcohol to be absorbed into the bloodstream slowly so that your BAC will only rise slowly, your best bet is to keep the alcohol in the stomach for as long as possible. This actually can be done. There is a valve between the stomach and the small intestine called the pyloric valve, and when this valve is closed the alcohol will stay in the stomach. This valve stays closed when the stomach is full of food. So this is why eating a full meal helps keep you from becoming rapidly intoxicated. Fatty foods and heavy foods tend to stay in the stomach longer than vegetables or sugars. Bluesman Charlie Patton spoke the truth when he said "If you eat a lot of fat meat you don't get so drunk." This was his formula for maintaining when he played at parties where the booze flowed all night long.What You Drink Does Matter!!
Some people say that alcohol is alcohol and it doesn't matter what you drink. The actual fact--as I am sure that many of us know from experience--is that it makes a great deal of difference what one drinks. This is true for several reasons.Alcohol Concentration: Many people find that they get much more intoxicated when drinking straight vodka than they do when drinking beer. This is because they get a lot more alcohol in their bodies in a lot shorter period of time when drinking the vodka. As a general rule of thumb the less concentrated the alcohol in a drink the less alcohol one will put into their body per hour.
Flavor: People also tend to drink strongly flavored drinks more slowly than tasteless drinks. So most people will get more alcohol into their system per hour when drinking vodka than they will when drinking whiskey.
Carbonation: Carbonation speeds the absorption of alcohol into the bloodstream. People drinking carbonated drinks will become intoxicated more quickly and achieve higher BACs than people dinking the same amount of alcohol per hour in the form of non-carbonated drinks. There is, however, a trade-off here because many people drink carbonated drinks more slowly than non-carbonated drinks.
Diet Soda: Diet soda interacts with alcohol too, so people who drink mixed drinks made with diet soda will become intoxicated more quickly and achieve higher BACS than people drinking identical drinks made with regular soda. Researchers in Adelaide, Australia found that the stomach emptied into the small intestine in 21.1 minutes for the people who drank mixed drinks made with diet soda. When people drank drinks made with regular soda, the stomach emptied in 36.3 minutes (P < .01). Peak blood alcohol concentration was 0.053 g% for the diet drinks and 0.034 g% with the regular drinks.
Beware Mixing Alcohol with Your Medications
The HAMS web site has a complete listing of alcohol-medication interactions here:Alcohol-related Drug Interactions
You should check this reference if you have any concerns about the interaction of a medication which you are taking with alcohol. Just for a quick reference we will note here some very common Over The Counter (OTC) and prescriptions medications and a few other substances which you should be very cautious about mixing with alcohol. Some of them may surprise you.
Aspirin: For some reason we are not quite sure of aspirin appears to block the action of alcohol dehydrogenase. What this means is that if you take aspirin before drinking you will became much more intoxicated on a much smaller dose of alcohol than usual. It is generally recommended that you do not take aspirin for around six hours before drinking alcohol. If you have taken aspirin before drinking be cautious and try to limit your alcohol intake as much as possible.
Cayenne pepper: Cayenne pepper dilates the blood vessels and apparently leads higher BACs and more exposure of the brain to alcohol. In short if you drink alcohol while ingesting a lot of cayenne pepper you will become much drunker than usual. Avoid red pepper vodka!
Tylenol (acetaminophen, paracetamol): Even by itself Tylenol can cause liver failure. Combining Tylenol with alcohol is a horrible one two punch to the liver. If you love your liver then don't take Tylenol or Tylenol PM or anything else containing acetaminophen with alcohol or when you are hungover. Else you might as well fry up your liver with onions!!
Ambien: mixing alcohol with ambien is just about a sure recipe for a blackout or a brownout. People who mix the two also often report sleepwalking or even sleep eating. Best to take one or the other and not mix them together.
Narcotic painkillers: Another recipe for blackout and disturbed behavior. Avoid mixing alcohol with Percocet, percodan, vicodin, oxycontin, codeine, morphine or any other narcotic pain killers.
Benadryl (diphenhydramine), Dramamine (dimenhydrinate), and Unisom Nighttime (doxylamine): Mixing alcohol with any antihistamine which causes drowsiness will definitely enhance the feeling of drowsiness many times over. All OTC sleep aids consist of one of the three above named antihistamines. Mixing them with alcohol is not medically dangerous, but beware of the added drowsiness.
The Effect of Smoking Tobacco (Nicotine):
Cigarette smoking slows gastric emptying and as a consequence delays alcohol absorption.Routes of Alcohol Ingestion
The only normal route of ingesting alcohol is drinking it--but this is not the only route possible. Other more exotic routes are used on occasion. Alcohol can be inhaled, absorbed through the skin, injected, or given as an enema. Let us take a look at each of these methods:Inhalation: AWOL (Alcohol With Out Liquid) is an alcohol inhalation device that has been released in the US and the UK. AWOL's manufacturers claim that when alcohol is vaporized and inhaled it can lead to intoxication as much as 10 times as quickly as drinking and allows one to sober up with no hangover in an equally rapid time frame. Doctors are still debating the safety of AWOL. At least 22 states in the US have banned AWOL.
Injection: Some scientific researchers give alcohol injections to research subjects when they wish to bypass the stomach. It was the comparison of the effects of injected alcohol with orally ingested alcohol which led scientists to conclude that women have less alcohol dehydrogenase in their stomachs than men do. Self-administration of alcohol by injection is extremely dangerous and should never be attempted. The risk of death by alcohol poisoning is extremely high.
Alcohol enema: This is another rather dangerous and sometimes deadly form of alcohol administration. If the internet is to be believed then alcohol enemas are not uncommon at sex parties. A beer enema might be safe enough. However the simple fact is that alcohol is absorbed very rapidly through the large intestine and the rectum and there are no enzymes here to break it down. Thus the same dose of alcohol given by enema will produce a much higher BAC than if one drinks it. There was a famous case of death by sherry enema in Texas where the wife was acquitted of murder charges. And a vodka enema is silent but deadly for sure.
Transdermal: Alcohol can also be absorbed through the skin although this is quite a slow and impractical method of ingesting it.
Why Alcohol Has a Steady State Metabolism Rather Than a Half Life
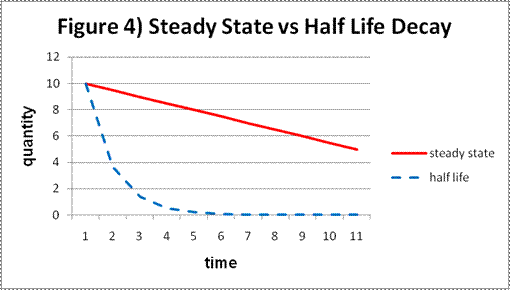
When a drug like valium is broken down by the human body the resultant metabolites are harmless. It is for this reason that drugs like valium are broken down as quickly as the body can process them--and hence they have a half life. The half life of valium is 35 hours on the average. This means that if you take a 10 mg dose of valium, then 35 hours later half of it will have been metabolized and only 5 mg will remain. In another 35 hours half of this will be metabolized and only 2.5 mg will remain and so on. When we plot the metabolism of valium on a graph we get an exponential curve--in other words--drugs which have a half life have an exponential rate of decay. Chemists refer to this as a First Order Reaction.Alcohol, on the other hand, shows a steady state metabolism not an exponential metabolism. The body of the average human metabolizes around 13 ml of alcohol per hour regardless. When we plot the metabolism of alcohol on a graph we get a straight line--in other words the rate of decay of alcohol is linear. Chemists refer to this as a Zero Order Reaction. The reason why alcohol has a steady state metabolism rather than a half-life metabolism is because the primary decay product of alcohol metabolism--acetaldehyde--is poisonous. The body must eliminate the acetaldehyde produced by the breakdown of alcohol before any more alcohol can be processed in order to avoid acetaldehyde poisoning. This slows down the rate of alcohol metabolism to a Zero Order Reaction rather than a First Order Reaction.
Figure 4 graphically illustrates the difference between steady state metabolism and half life metabolism.
Why Do Humans Have a Way To Break Down Alcohol?
Practically every animal from the fruit fly to the elephant has a way to break down ethyl alcohol because ethyl alcohol is found everywhere in nature. Every time you eat a piece of fresh fruit, drink a glass of fresh orange juice, or have a slice of freshly baked bread then chances are that you are getting trace amounts of alcohol along with it. It is not uncommon to see intoxicated birds which have eaten fermented fruit. Monkeys are known to seek out fermented fruit for the intoxicating effect and Indian elephants have been known to break into breweries or wineries to drink up what is stored there.Not only are we constantly ingesting alcohol along with the food we eat, our own bodies produce alcohol as a part of the digestive process. Our digestive tracts contain millions of micro-organisms which are necessary for us to properly digest our food. Among these micro-organisms are yeasts which produce alcohol from sugars within our own bodies.
With alcohol so omnipresent in nature it is necessary that animals have a way to break alcohol down, otherwise it would just accumulate in the body and no animal could function properly because the animals would always be constantly intoxicated.
Other alcohols such as methyl alcohol (wood alcohol) and isopropyl alcohol (rubbing alcohol) do not normally occur in nature. This is why we do not have a mechanism to break them down and why they are poisonous.
Poisonous Alcohols
The difference between wood alcohol--also known as methyl alcohol or methanol--and ethanol is that wood alcohol has one less carbon and two less hydrogen atoms. The chemical formula for ethanol is C2H6O whereas the formula for methanol is CH4O. Alcohol dehydrogenase converts methanol into formaldehyde (CH2O) and aldehyde dehydrogenase turns this formaldehyde into a formic acid radical (CH2O-). Both formaldehyde and formic acid are highly poisonous and quickly lead to blindness and death.Another highly poisonous alcohol is ethylene glycol (C2H6O2) which is used in antifreeze. A metabolite of ethylene glycol is the highly poisonous oxalic acid.
Rubbing alcohol (C3H8O)--also known as isopropyl alcohol--is more poisonous than ethanol but not as poisonous as methanol. Some chronic alcoholics turn to drinking rubbing alcohol when ethanol is unavailable--and some even come to prefer it.
Alcohol and Blood Sugar
Although alcohol may cause a slight rise in blood sugar levels when initially ingested--the overall effect of alcohol is to cause a drop in blood sugar. The more you drink the more the blood sugar drops. Eating before, during or after drinking can help to alleviate this blood sugar drop somewhat. Drinks with lots of carbs like beer or mixed drinks with sugary mixers can lead to blood sugar spikes preceding the blood sugar drop.Because of alcohol's effect on blood sugar people with diabetes are recommended to have no more than one or two standard drinks per day and to avoid drinks high in carbs. Untreated diabetes can lead to severe consequences including blindness, amputation of limbs affected by gangrene and even death--so diabetics are recommended to be especially cautious about their alcohol intake.
REFERENCES:
Anderson C, Andersson T, Molander M. (1991). Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Dermato Venereologica.71(5):389-93.ABSTRACT
Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. (1990). High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. New England Journal of Medicine. Jan 11;322(2):95-9.
ABSTRACT
Johnson RD, Horowitz M, Maddox AF, Wishart JM, Shearman DJ. (1991). Cigarette smoking and rate of gastric emptying: effect on alcohol absorption. BMJ. Jan 5;302(6767):20-3.
ABSTRACT
MSNBC (2007). Elephants electrocuted in drunken rampage
Retrieved June 15, 2009
http://www.msnbc.msn.com/id/21432722/?GT1=10450
Reuters. Charges dismissed in Texas sherry enema death - By Erwin Seba
Wed Oct 3, 2007 7:18pm EDT.
Retrieved June 13, 2009.
http://www.reuters.com/article/newsOne/idUSN0325982220071003
Wu KL, Chaikomin R, Doran S, Jones KL, Horowitz M, Rayner CK. (2006). Artificially sweetened versus regular mixers increase gastric emptying and alcohol absorption. The American Journal of Medicine. Sep;119(9):802-4.
AWOL - Alcohol Without liquid
http://en.wikipedia.org/wiki/Alcohol_without_liquid
Blood alcohol concentration. The Psychology Wiki.
http://psychology.wikia.com/wiki/Blood_alcohol_concentration
Alcohol - Drugtext
http://www.drugtext.org/Psychopharmacology/alcohol.html
Alcohol, Chemistry and You
Metabolism of Ethyl Alcohol in the Body
Dr. Bill Boggan
http://www.hamsnetwork.org/boggan/
Alcohol flush reaction
http://psychology.wikia.com/wiki/Alcohol_flush_reaction
Aldehyde dehydrogenase
http://en.wikipedia.org/wiki/Aldehyde_dehydrogenase
RCSB Protein Data Bank
Alcohol Dehydrogenase
January 2001 Molecule of the Month
by David S. Goodsell
http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb13_1.html
Hangover
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Hangover
Acetaldehyde dehydrogenase
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Acetaldehyde_dehydrogenase
Alcohol dehydrogenase
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Alcohol_dehydrogenase
Alcohol Metabolism Effects - Elmhurst College
http://chemistry.elmhurst.edu/vchembook/642alcoholmet.html
Overview: How Is Alcohol Metabolized by the Body?
http://pubs.niaaa.nih.gov/publications/arh294/245-255.htm
Role of Acetaldehyde in Mediating the Pharmacological and Behavioral Effects of Alcohol
http://pubs.niaaa.nih.gov/publications/arh294/258-265.htm
UpToDate. Patient information: Diabetes mellitus type 2: Alcohol, exercise, and medical care
Accessed June 15, 2009
http://www.uptodate.com/patients/content/topic.do?topicKey=~btiSIVAV2lvR5
American Diabetes Association. Alcohol.
Accessed June 15, 2009



